
George Bloom

Office Address: Gilmer 420
Education
B.A., University of Pennsylvania, 1973
Ph.D., University of Pennsylvania, 1979
Research Interests
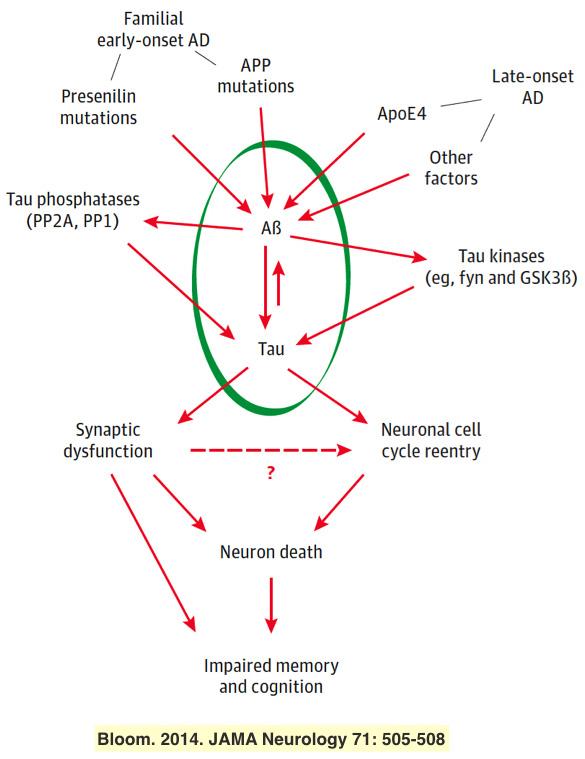
Research in our laboratory is now focused primarily on Alzheimer’s disease (AD). The histopathological hallmark of AD is the presence in brain of extracellular plaques of ß-amyloid peptide fibrils, and intraneuronal neurofibrillary tangles, which are filaments composed of the protein, tau. Despite the conspicuous appearance of plaques and tangles, a growing body of evidence points to their building blocks, ß-amyloid and tau oligomers, as being the toxic molecular species that cause AD. For example, we have found that tau expression is required for several adverse effects of ß-amyloid oligomers on neurons, including microtubules loss, ectopic re-entry into the cell cycle and cytotoxicity. The goals of our work are to decipher the metabolic links that connect ß-amyloid and tau to damage neurons, to define the structures and pathological properties of various types of ß-amyloid and tau oligomers, and to develop effective therapeutic and diagnostic tools for AD.
Representative Publications
Norambuena A, Sagar VK, Wang Z, Raut P, Feng Z, Wallrabe H, Pardo E, Kim T, Alam SR, Hu S, Periasamy A and Bloom GS. 2024. Disrupted Mitochondrial Response to Nutrients is a Presymptomatic Event in the Cortex of the APPSAA Knock-In Mouse Model of Alzheimer’s Disease. Alzheimer’s & Dementia https://doi.org/10.1002/alz.14144.
Sun X, Eastman G, Shi Y, Saibaba S, Oliveira AK, Lukens JR, Norambuena A, Mandell JW and Bloom GS. 2023. Structural and functional damage to neuronal nuclei caused by extracellular tau oligomers. Alzheimer’s & Dementia: DOI: 10.1002/alz.13535.
Best MN, Lim Y, Ferenc NN, Kim N, Min L, Wang DB, Sharifi K, Wasserman AE, McTavish SA, Siller KH, Jones MK, Jenkins PM, Mandell JW and Bloom GS. 2023. Extracellular Tau Oligomers Damage the Axon Initial Segment. Journal of Alzheimer's Disease: https://doi.org/10.3233%2FJAD-221284.
Rajbanshi B, Guruacharya A, Mandell JW and Bloom GS. 2023. Localization, induction, and cellular effects of tau phosphorylated at threonine 217. Alzheimer’s & Dementia https://doi.org/10.1002/alz.12892.
Norambuena A, Sun X, Wallrabe H, Cao R, Sun N, Pardo E, Shivange N, Wang DB, Post LA, Ferris HA, Hu S, Periasamy A and Bloom GS. 2022. SOD1 mediates lysosome-to-mitochondria communication and its dysregulation by amyloid-β oligomers. Neurobiology of Disease 105737 https://doi.org/10.1016/j.nbd.2022.105737.
Norambuena A, Wallrabe H, Cao R, Bigler Wang D, Silva A, Svindrych Z, Periasamy A, Hu S, Kim DY, Tanzi R and Bloom GS. 2018. A Novel Lysosome-to-Mitochondria Signaling Pathway Disrupted by Amyloid-β Oligomers. EMBO Journal 37: e100241. PMCID: PMC6236329.
Kodis EJ, Choi S, Swanson E, Ferreira G and Bloom GS. 2018. NMDA receptor-mediated calcium influx connects amyloid-b oligomers to ectopic neuronal cell cycle re-entry in Alzheimer’s disease. Alzheimer’s & Dementia 14: 1302-1312. P PMID: 30293574.
Norambuena A, Wallrabe H, McMahon L, Silva A, Swanson E, Thomas S, Baerthlein D, Kodis E, Oddo S, Mandell JW and Bloom GS. 2017. mTOR and Neuronal Cell Cycle Re-entry: How Impaired Brain Insulin Signaling Promotes Alzheimer’s Disease. Alzheimer’s & Dementia 13: 152-167. PMCID: PMC5318248.
Bloom GS. 2014. Amyloid and Tau: the Trigger and Bullet in Alzheimer's Disease Pathogenesis JAMA Neurology 71: 505-508. PMID 24493463.
Nussbaum JM, Schilling S, Cynis H, Silva A, Swanson E, Wangsanut T, Tayler K, Wiltgen B, Hatami A, Rönicke R, Reymann K, Hutter-Paier B, Alexandru A, Jagla W, Graubner S, Glabe CG, Demuth H-U and Bloom GS. 2012. Prion-Like Behavior and Tau-dependent Cytotoxicity of Pyroglutamylated Amyloid-β. Nature 485: 651-655. PMCID: PMC3367389.